Electrolytic Removal of Rust - a Detailed Illustrated Tutorial, p.34
7. How To Set Up Multiple-Relic Electrolysis: Attaching Iron Relics to Cathodes and Suspending Iron Parts in Electrolyte
2) Attaching Iron Relics to Their Wire Cathodes
As described in details in the "Preparing an Artifact for Electrolytic Derusting" section on page 18, before attaching an iron artifact to the cathode wire, you need to file, scrape or grind the rust off the contact point 1 on the artifact where a good contact between the cathode wire and the artifact must be established.
Also, in order to ensure completion of the electric circuit, i.e. ability of dc current to run through the iron object being derusted, you need to provide a good contact between the electrolyte and the artifact's part to be submerged. So you need to file the rust off another contact point - point 2, that should be located in the submerged area.
Thus, before being attached to the cathode grid on the multiple-relic hanger, each of six iron relics must have two contact points. While attaching the artifacts to their wire cathodes, make sure that none of the long-shaped artifacts, when they are suspended, would hang too close to the bottom of the container. Otherwise these artifacts would touch the sludge that would eventually accumulate on the bottom during electrolysis.
Rusty Iron Relics Before Being Attached to a Hanger

Attach each artifact to its cathode wire at the contact point 1. While winding the wire around the artifact, make sure that the wire has a metal-to-metal contact with the iron surface. Make the connection sufficiently tight by twisting the cathode wire around itself as shown on a photo below.
Tight Connection to Wire Cathode

Attach the rest of relics to their wire cathodes the same way as described above.
All Iron Artifacts Are Attached to Multiple-Part Hanger and to Cathode Electrical Grid

Suspending Iron Relics from Multiple-Part Hanger
To avoid any mishaps during the process of the multiple-part electrolysis, you should follow these simple rules:
1) The suspended iron relics should be as close to the anodes as possible but NOT touching them, otherwise the short-circuiting will occur. However, the iron relics and the anodes should not be positioned too far apart, otherwise derusting them will take forever. To speed up the process, additional anodes can be placed in the container, as many as needed.
A Stainless Steel Anode Is Added to Multiple-Part Setup (Do Not Mind the Snow Falling!)
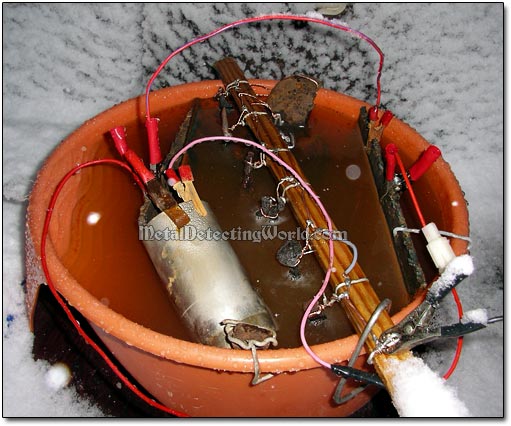
The top priority should be given to safe placement of the anodes so that they would not accidentally touch or fall over the relics. Each anode MUST BE secured to the plastic container's rim with a bracket, tape or clamps to ensure its immovability (see details in the "Proper Placement of Electrodes" section on page 19). I used another dug fragment of aluminum wire to make a simple stopper-bracket.
A Bracket Prevents Movements of Anode
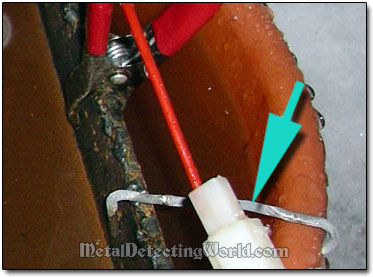
The non-conductive part of the multiple-relic hanger should be also secured to the container's rim by means of a stopper-bracket or a clamp as well. But you should do it at last - only AFTER everything is properly connected to work.
A Stopper-Bracket Prevents Movements of Multiple-Relic Hanger
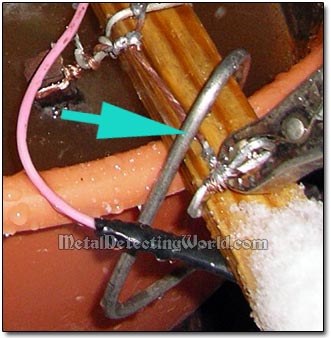
2) NONE of six cathode wires should be immersed into electrolyte, otherwise the current will flow through the immersed cathode - the less resistant electrical conductor, into the electrolytic solution; avoiding the iron part to be derusted. In other words, the clean surface of the wire cathode would be wasting electricity splitting water while the rusted artifact receiving the least amount of current is slowly cleaned.
Cathode Wires Are Kept Out of Electrolyte

The only drawback would be that an area of the iron object, to which the cathode wire is adjacent, would not be electrolyzed, and, therefore, the iron object would not get fully derusted in one session. So you will have to run another session to derust the remaining rusty area. And if you process it a little longer than necessary, there will be no visible difference between the two derusted areas.
I found out from my experiments with electrolysis that derusting an iron relic in two sessions still takes less time than electrolyzing the relic along with the immersed cathode at once. Some enthusiasts might argue against that, but there are no two electrolytic setups or operational conditions the same in the world, so there is no point in arguing about it.
One can employ whatever method works for him the best. Another reason I keep the cathode out of the electrolyte that I do not want the cathode wires to be contaminated with toxic hexavalent chromium that appears in the electrolyte when the stainless steel anodes are used.